Lyophilization Technology
LYOVAC® Small-Scale Freeze Dryers
Extending its range of LYOVAC® freeze dryers, GEA now offers both integrated and standalone equipment for the production of small-scale batches and formulation or process development
Pilot Freeze Dryer LYOVAC® FCM 10-P
The highly flexible standalone FCM 10-P freeze dryer from GEA has been designed for the production of small-scale batches and formulation/process development. Also available as a wall-mounted option, this two-chamber unit is configured to industry standards.
With a capacity of 10 kg and shelf space of 0.63 m², the FCM 10-P also benefits from easy-access doors and spare flanges for validation and/or additional equipment.
Using the same FALCO control system as the production unit, allowing direct recipe transfer during scale-up, the FCM 10-P is GLP/GMP-compliant.
Available options:
- IQ/OQ documentation and test performance
- Wireless temperature sensors
- Sterilization by VHP (VAPOVAC™) or steam depending on the availability of utilities
- Process development service
- LYOSPARK© controlled nucleation
- LYOPLUS© mass spectrometer for silicone oil detection or alternative end-point detection

| FCM10-P | |
| Capacity | 0,63m² shelf area |
| 4 shelves | |
| 10 kg in in 24 h | |
| Possible Load | 2608 of 2R vials |
| 1352 of 6R vials | |
| 708 of 20R vials |
VarioSys®
A combination of isolator and machine modules, VarioSys® provides a quick and flexible way to process liquid, powder or lyophilized product in vials, ampules, syringes or cartridges. Operating in a Grade C environment, filling and capping systems can be quickly and easily interchanged, reducing in both footprint and equipment costs.
Downloads
منتجات ذات صلة
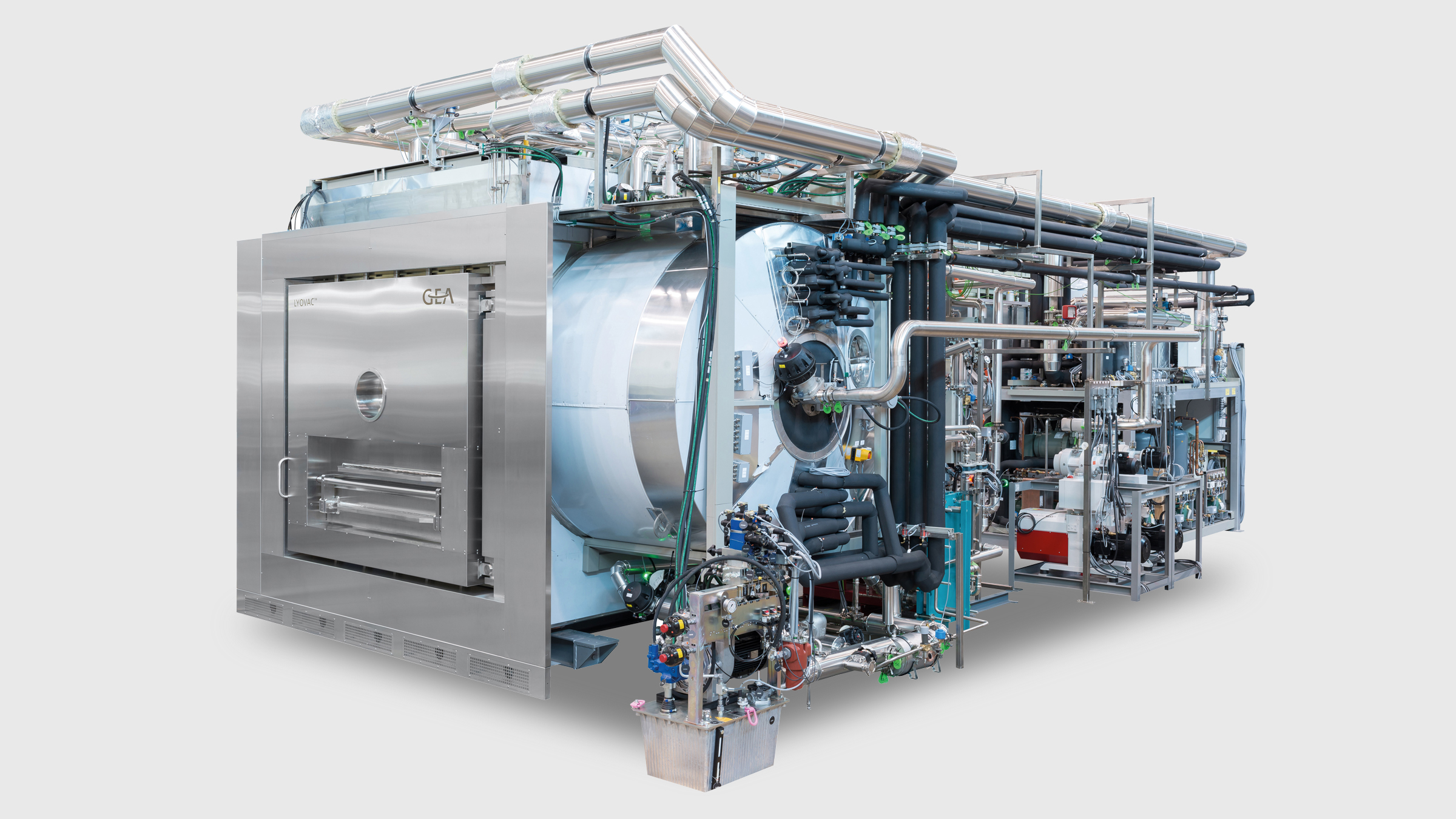
LYOVAC® Pharma Freeze Dryer
GEA’s LYOVAC® industrial-scale freeze dryers are built to customer specifications and are available with production areas of 1–60 m² and condenser capacities of more than 1000 kg.

SMART LYO® Pharma Freeze Dryer
SMART LYO™ Freeze Dryers help reduce the cost of freeze drying while maintaining quality and performance, making validation and documentation easier and reducing delivery times.

Process Analytical Technology (PAT) for Lyophilization: LYOPLUS®
LYOPLUS™ mass spectrometer is a multi-purpose measurement device for pharmaceutical freeze dryers.

ALUS® Load & Unload of Lyophilizers
ALUS® Automatic Load & Unload Systems minimize the risk of operator/product contamination while loading and unloading a freeze dryer.
رؤى GEA
All pharmaceutical freeze-drying vials are the same! Aren't they?
Whether it’s a fad or the future, 100% vial traceability is becoming an increasingly important consideration in the pharmaceutical freeze drying industry. Keeping a close eye on developments is GEA. We’re investigating possible solutions and, what’s more, we have the experience, expertise and know-how to implement them.
البيرة الخالية من الكحول: إنهم يريدون كل شيء - إنهم يريدون أقل
كان هناك وقت حيث من النادر استخدام عبارتي "بيرة خالية من الكحول" و "طعمها جيد" معًا في الجملة نفسها، خاصة من قبل المستهلكين. ولكن قطعت البيرة منخفضة الكحول والبيرة الخالية من الكحول شوطًا طويلًا - وأصبح الكثير منها الآن مشروبات منعشة في حد ذاتها - بفضل جزء صغير من تكنولوجيا GEA.
Innovating patient care with aseptic spray drying
At GEA, our commitment to engineering for a better world fuels our pursuit of innovative solutions that enhance patient care and safety. One of our most promising ventures in recent years is aseptic spray drying – a technology that promises to revolutionize pharmaceutical manufacturing.

