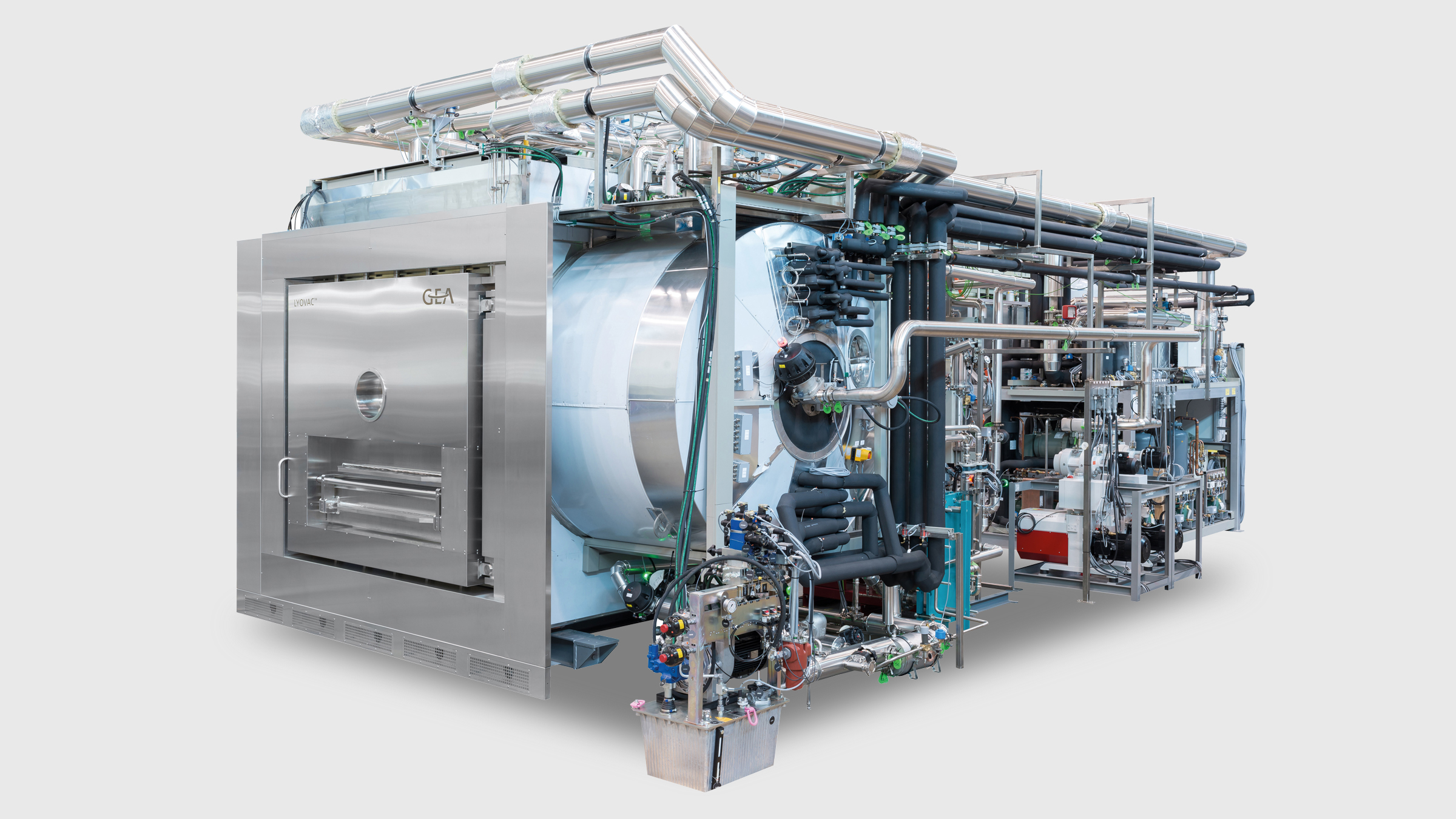
Lyophilization Technology
LYOVAC® Pharma Freeze Dryer
GEA’s LYOVAC® industrial-scale freeze dryers are built to customer specifications and are available with production areas of 1–60 m² and condenser capacities of more than 1000 kg.
LYOVAC®freeze dryers can be arranged on one or multiple floors, such that the condenser is typically situated under the drying chamber and ensures both easy access to the machine parts and complete separation between the cleanroom and the technical area.
In a single-floor setup, the orientation of the condenser can be on the side or behind the drying chamber. No matter what the size or the arrangement, the unique shelf design ensures high drying rates and uniform results. In addition, all LYOVAC®freeze dryers can be integrated with our Automatic Loading and Unloading System (ALUS®).
LYOVAC® is also available using GEA’s unique Fast Track manufacturing process. Depending on the complexity of the installation and based on previously completed projects, it is possible to have a system up and running in less than a year.
Invented by GEA in 1980s, ALUS®minimize the risk of operator/product contamination while loading and unloading a freeze dryer, offering shorter processing times, improved productivity and reduced operational costs. We were also the first company to provide an isolated ALUS in the 1990s.
Given the absolute requirements to minimize contamination, eliminate operator exposure and protect the product, further containment options include isolators, conventional cleanrooms or both active and passive Restricted Access Barrier Systems (RABS).
Key features
- GMP equipment
- CIP/SIP with quick subprocess turnaround times
- Solvent processing capabilities
- Highly potent processing capabilities
- Refrigeration using either compressors, LN2, natural refrigerants, etc.
- State-of-the-art automation options with virtual servers
- LYOPLUS®
- LYOSPARK® controlled nucleation
- Fast track delivery system
- In-house ALUS® with integrated FATs possible
- In-house shelf manufacturing
Process Optimization
LYOSPARK® Controlled NucleationA novel approach to simple, reliable and easily controlled nucleation during industrial freeze drying
The lyophilization process comprises three main stages: freezing (solidification), primary drying (ice sublimation) and secondary drying (moisture desorption). The nucleation of ice crystals (the initial stage of ice formation during freezing) is a naturally spontaneous and protracted process. As such, forcing ice crystals to nucleate at a specific temperature, rate and time can improve the homogeneity of the batch and reduce the cycle duration.
Historically, because of the random nature of the nucleation process, lyophilization has been difficult to control. For a typical pharmaceutical formulation filled in vials, for example, crystals might form at temperatures ranging from –5 to –20 °C).
As the nucleation temperature has a direct impact on the developing ice structure and crystal size — as well as the appearance of the dried product cake — it can negatively influence the product’s Critical Quality Attributes (CQAs), such as residual moisture or dissolution behavior.
A patented and proven technology from GEA, which is fully compatible with all our currently available freeze dryers, provides a novel and scalable method to control the nucleation process without having to develop new pharmaceutical formulations.
Simultaneous Crystal Nucleation
LYOSPARK® enables the user to control the temperature at which nucleation crystals form inside the product, thereby ensuring that, at any time, every vial in the chamber is subjected to the same process conditions, irrespective of its specific location on the shelf.
Combining LYOSPARK® technology with conventional freeze-drying processes is very simple. The vials are loaded into the freeze dryer as usual and cooled to the optimal (product dependent) nucleation/cooling temperature. As soon as the nucleation temperature is reached, the chamber is vented with gas from the freeze dryer’s condenser.
A filter-equipped cooling trap is mounted on the chamber. Ice crystals form on the cooled surface of the trap and are entrained in the gas stream during venting. Inside the supercooled product solution, the ice crystals undergo immediate and homogenous nucleation in each vial.
As the formation of ice crystals at higher temperatures results in larger pores, drying times can be shortened. Furthermore, LYOSPARK® offers better scalability and higher throughput speeds than conventional systems, resulting in optimized performance and productivity.
Process Optimization
LYOSPARK®, which eliminates issues such as the denaturation of active ingredients or unpredictable scale-up, also provides a number of technical advantages, including improved product quality, better vial uniformity per batch and, depending on the product, a more homogenous, aesthetically pleasing cake. It’s also recommended for highly potent applications as no toxic gas is released to the environment.
In summary, LYOSPARK® controlled nucleation technology facilitates uniform ice crystal formation in production-scale freeze dryers with a minimum degree of supercooling. This is reflected in more consistent and larger ice crystal sizes with a more open product structure. As a result, faster drying and reconstitution times can be achieved.
QbD-compliant, GEA’s LYOSPARK® delivers a more uniform, higher quality product, reduces process times and provides a cost-effective solution to controlled nucleation.
Advantages at a glance
- Larger ice crystals
- Shorter drying times
- Better process control
- Better reconstitution
- Less vial cracking
- Less stress on biological agents
This cost-efficient technology will be offered as an option for all GEA freeze dryers and will also be available as a retrofit upgrade for existing plant.
Downloads
Verwandte Produkte

LYOVAC® Small-Scale Freeze Dryers
Extending its range of LYOVAC™ Freeze Dryers, GEA now offers both integrated and standalone equipment for the production of small-scale batches and formulation or process development.

Process Analytical Technology (PAT) for Lyophilization: LYOPLUS®
LYOPLUS™ mass spectrometer is a multi-purpose measurement device for pharmaceutical freeze dryers.

SMART LYO® Pharma Freeze Dryer
SMART LYO™ Freeze Dryers help reduce the cost of freeze drying while maintaining quality and performance, making validation and documentation easier and reducing delivery times.

ALUS® Load & Unload of Lyophilizers
ALUS® Automatic Load & Unload Systems minimize the risk of operator/product contamination while loading and unloading a freeze dryer.
Zugehörige Videos
Virtual Twin of a LYOVAC Freeze Dryer
GEA Insights
Gefriertrocknung in der Pharma-Branche: Alle Vials sind gleich! Oder?
Nur eine Zeiterscheinung oder die Zukunft? In jedem Fall wird die hundertprozentige Nachverfolgbarkeit von Vials für pharmazeutische Unternehmen, die Gefriertrocknung einsetzen, ein zunehmend wichtiges Thema. Wir bei GEA verfolgen diese Entwicklung aufmerksam und prüfen mögliche Lösungen. Vor allem aber verfügen wir über die Erfahrung, den Sachverstand und das Know-how, sie umzusetzen.
Es geht auch ohne: Alkoholfreies Bier kommt an
Es gab Zeiten, in denen die Worte „alkoholfreies Bier“ und „schmeckt gut“ kaum je im selben Atemzug genannt wurden – schon gar nicht von den Konsumenten. Doch alkoholarme und alkoholfreie Biere haben eine enorme Entwicklung hingelegt und sind heute vielfach als Erfrischungsgetränke etabliert. Nicht zuletzt dank der Technologie von GEA.
Innovating patient care with aseptic spray drying
At GEA, our commitment to engineering for a better world fuels our pursuit of innovative solutions that enhance patient care and safety. One of our most promising ventures in recent years is aseptic spray drying – a technology that promises to revolutionize pharmaceutical manufacturing.