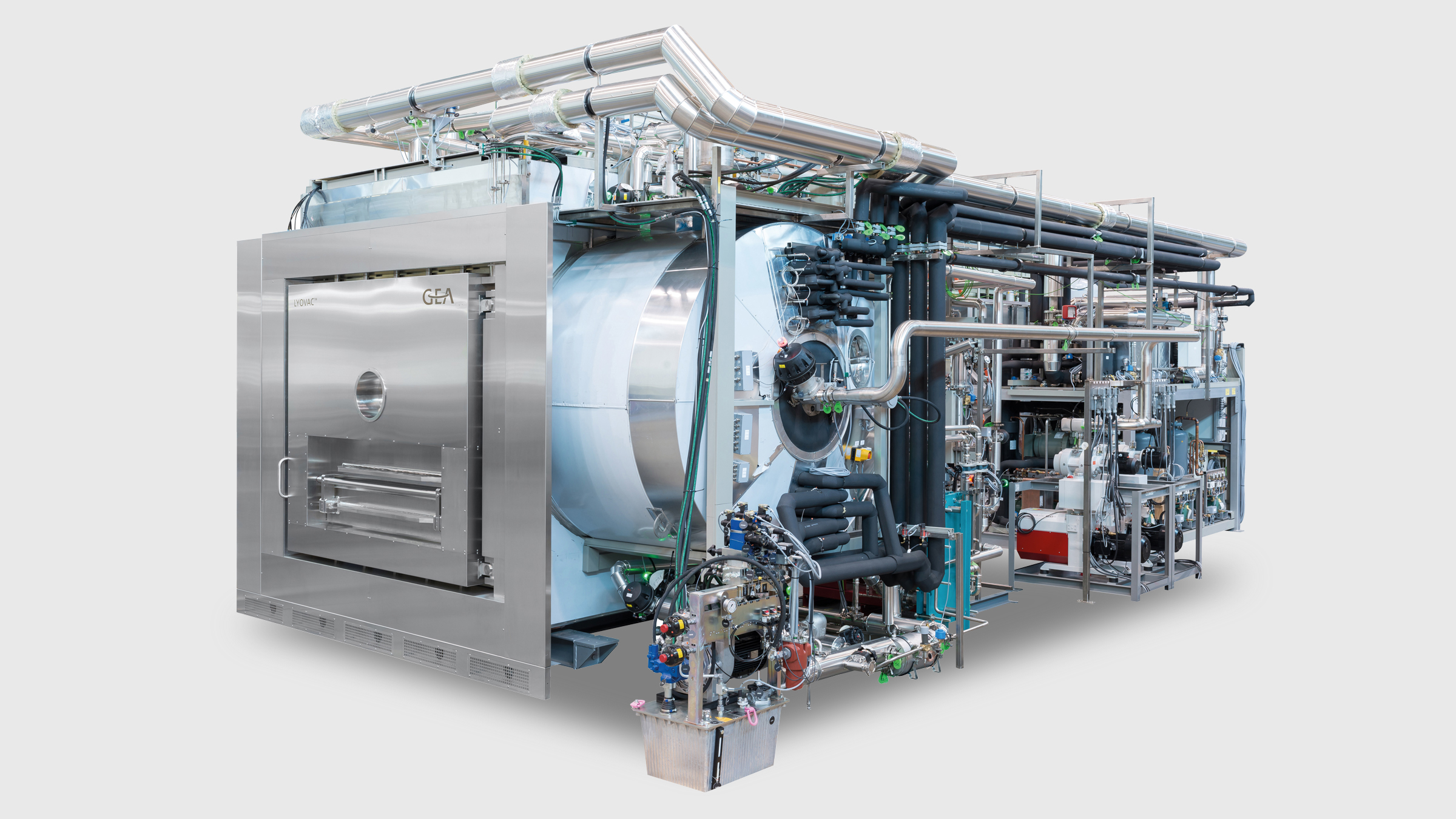
LYOVAC® Freeze dryers for small scale production modules
For flexible product development and small-scale production, including the use of nests and tubs, the FCM-I range of LYOVAC® Freeze Dryers from GEA provides reliable, safe and cost-effective productivity and price/performance excellence.
Designed to comply with the most stringent good manufacturing practice (GMP) standards, the LYOVAC® FCM 25-I, FCM 75-I and FCM 150-I freeze dryers are a unique and efficient combination of proven technology and individual solutions that are customized to meet specific user requirements.
Integrated VarioSys® Isolator
Built with operator safety in mind, full isolator protection for processes involving potent or toxic substances is available, and each unit is supplied with an integrated L-flange for the modular isolator system VarioSys®.
Supplied in three sizes (1.5 m², 4.5 m² and 9.9 m²) with nominal condenser capacities of 25 kg, 75 kg and 150 kg, the smaller FCM 25-I machine can process up to 6500 vials (2 mL) per batch, whereas the larger FCM 75-I and FCM 150-I units are capable of handling more than 20,000 and 42,000 similarly sized vials, respectively. An automatic loading and unloading system (ALUS®) is available.
| FCM 25-I | FCM 75-I | FCM 150-I | |
|---|---|---|---|
| Capacity | |||
| Shelf area | 1.5 m² | 4.5 m² | 9.9 m² |
| Shelves | 5 | 5 | 7 |
| Kg ice/24 h | 25 kg | 75 kg | 150 kg |
| Possible Load | |||
| 2R vials | 6523 | 20,039 | 42,406 |
| 6R vials | 3388 | 10,506 | 22,295 |
| 20R vials | 1778 | 5583 | 11,900 |
VarioSys: Evonik Case Study
The LYOVAC® freeze dryer offer the following features and benefits:
- compact footprint with a belly condenser design
- suitable for Grade C environments
- LYOSPARK® controlled nucleation
- LYOPLUS® silicone oil detection
- Fast turnaround through defrost/CIP/SIP
- alternative end-point detection is also available.
Plus, fully compatible with toxic, potent and volatile products, the FCM units are ATEX-compliant and designed for easy scale-up. Partial loading is also possible for variable batch sizes.
Operating in a Grade C environment, other modules, such as filling and capping systems, can be quickly and easily interchanged, resulting in both a dramatic reduction in footprint and equipment costs.
Available for rapid delivery — with low infrastructure requirements — for multiple product and/or delivery device lines, the modules offer an ideal solution for vial, ampoule, syringe and cartridge processing, as well as liquid, powder or lyophilized form applications.
Downloads
Verwandte Produkte
LYOVAC® Pharma Freeze Dryer
GEA’s LYOVAC® industrial-scale freeze dryers are built to customer specifications and are available with production areas of 1–60 m² and condenser capacities of more than 1000 kg.

SMART LYO® Pharma Freeze Dryer
SMART LYO™ Freeze Dryers help reduce the cost of freeze drying while maintaining quality and performance, making validation and documentation easier and reducing delivery times.

Process Analytical Technology (PAT) for Lyophilization: LYOPLUS®
LYOPLUS™ mass spectrometer is a multi-purpose measurement device for pharmaceutical freeze dryers.

ALUS® Load & Unload of Lyophilizers
ALUS® Automatic Load & Unload Systems minimize the risk of operator/product contamination while loading and unloading a freeze dryer.
GEA Insights
Gefriertrocknung in der Pharma-Branche: Alle Vials sind gleich! Oder?
Nur eine Zeiterscheinung oder die Zukunft? In jedem Fall wird die hundertprozentige Nachverfolgbarkeit von Vials für pharmazeutische Unternehmen, die Gefriertrocknung einsetzen, ein zunehmend wichtiges Thema. Wir bei GEA verfolgen diese Entwicklung aufmerksam und prüfen mögliche Lösungen. Vor allem aber verfügen wir über die Erfahrung, den Sachverstand und das Know-how, sie umzusetzen.
Es geht auch ohne: Alkoholfreies Bier kommt an
Es gab Zeiten, in denen die Worte „alkoholfreies Bier“ und „schmeckt gut“ kaum je im selben Atemzug genannt wurden – schon gar nicht von den Konsumenten. Doch alkoholarme und alkoholfreie Biere haben eine enorme Entwicklung hingelegt und sind heute vielfach als Erfrischungsgetränke etabliert. Nicht zuletzt dank der Technologie von GEA.
Innovating patient care with aseptic spray drying
At GEA, our commitment to engineering for a better world fuels our pursuit of innovative solutions that enhance patient care and safety. One of our most promising ventures in recent years is aseptic spray drying – a technology that promises to revolutionize pharmaceutical manufacturing.



